The UAE Ministry of Health and Prevention have recalled products that cause damaging health effects.
The products include skin care products with whitening and pigmentation altering properties, pharmaceutical products that failed tests for international standards, dietary supplements and a batch of contact lenses which have a manufacturing defect.
Whitening products

The whitening products were found to contain hydrocarbon substances which are classified to be used only under medical prescription and must be registered at the Ministry.
The products also contained Hydroquinone and showed a high concentration of mercury. Hydroquinone is a drug used to treat skin spots and should only be bought with a prescription in pharmacies.
It can cause severe problems including redness, dryness and cracking of the skin. Mercury may also cause toxic effects, such as nausea, abdominal pain, muscle spasm, anaemia, as well as liver and nervous system problems.
Dietary Supplements
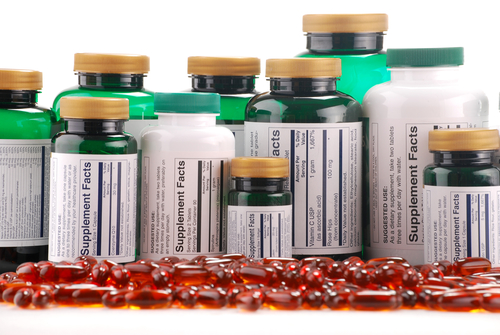
The ministry also warned against the use of dietary supplements that could pose a significant risk to patients with heart disease, arrhythmia or strokes, as well as other supplements that contain carcinogens, others with sexual stimulants that may cause a dangerous drop in blood pressure, and harm patients that have heart disease, diabetes, or dyslipidemia - especially those treated with nitrates.
It also warned against weight loss coffee bags which may cause an increase in blood pressure, among others.
Pharmaceutical products
Pharmaceutical products must melt within the necessary solubility period in order to be considered economically friendly.
Contact Lenses

Johnson & Johnson voluntarily recalled their contact lenses after reports that it caused unclear vision came to light.
The lenses are ACUVUE® OASYS® Brand Contact Lenses for ASTIGMATISM with HYDRACLEAR® PLUS with batch number ‘B00GW4Z’.
Always check the ingredients of the products before you use them.